We established that melanin-based nanoshells can be used in the protection of human beings and machinery from electromagnetic radiation, in particular, from ionizing radiation, and in energy conversion such as minimizing the losses during energy transfer.
Organization: Albert Einstein College of Medicine, New York, USA
Inventors: E. Dadachova PhD and A. Casadevall MD, PhD
Primary market: Aerospace, Defense, Electronics and Health/Medical
Technology Contact: J. Harb, Albert Einstein College of Medicine, New York, USA

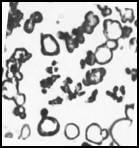

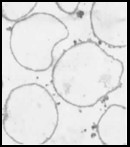
Melanin nanoshells can be made in different shapes and sizes. Size bar represents 1 micron.
IP description: Currently, there are no materials for protection from electromagnetic radiation like electronic pulses, microwave radiation, radiation from the wireless devices etc. Lead is used to shield only from ionizing radiation - it is heavy and toxic metal which makes shielding of the aircraft, personnel or patients cumbersome or impossible. The availability of light-weight radiation- and electron-resistant nanomaterials would allow protection of astronauts, pilots, military personnel as well as patients and personnel in heath care were different types of radiation are used. Also, electronic equipment could be protected without the loss of overall performance. Melanin is a high molecular weight pigment that is ubiquitous in nature. We established that melanin nanoshells protect against radiation and suggested that its protective efficacy is a function of its chemical composition and spatial arrangement in nanoshells. Mice fed with melanin nanoshells survived lethal doses (9 Gy) of ionizing radiation. The melanin nanocoatings could be used to cover computer drives, and computer chips in sensitive equipment on the ground and in the air to protect it from electronic pulses and radiation. Also melanin nanomaterials could be incorporated into the protective gear of astronauts, pilots, and military personnel to protect them from excessive radiation. The spin-off applications include protective creams, apparel and shields for patients and personnel in radiation oncology, interventional radiology and nuclear medicine. The interested companies could be Boeing, General Electric, Phillips, Siemens, Capintec and bio/nanotechnology companies.
Funding: We are looking for partners in Aerospace, Defense, Electronics and Health/Medical industry to develop jointly this versatile technology to fit their particular market needs.